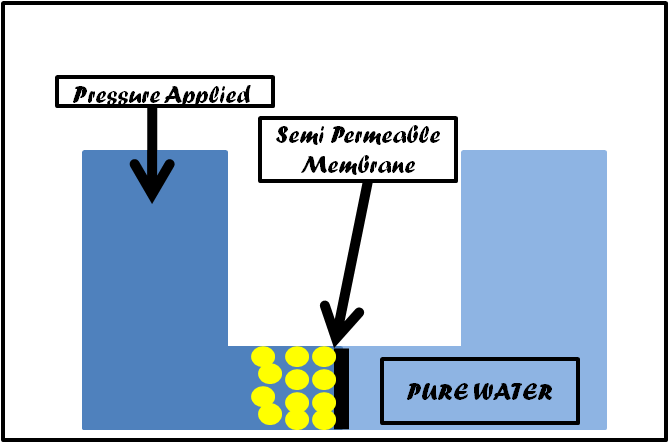
- The Reverse Osmosis Unit actually has many advantages and are good for you, the citizens!
- The Reverse Osmosis Unit actually filters tap water into pure water.
- This has many benefits as usually the tap water contains many harmful substances such as chlorine which can seriously damage your health over a period of time.
- Consumes no energy, which means it is good for the earth and ultimately also prolongs your lifetime! You can also repeatedly use it without worries.
- It is in your kitchen! You don't need the hassle trying to boil the water which also removes the oxygen which is good for your health!
- Free, there is no need to pay for filtering the water, however, paying is necessary for the set-up.
Reverse Osmosis is not only used in households but also in factories where they mass filter seawater, which is extremely large in quantity, into pure water for the general public to drink and consume.
Reverse Osmosis is used for many different purposes in factories:
- The first and most common usage: Water Purification. As said, thousands of plants are constructed so that there will be water supplied to the people.
- Supply of water to armies. America was the first country to use this technique. This was because they were the country who funded the research of Reverse Osmosis.
- Supply of water for reef aquariums. Organisms, both plants and animals can obtain detrimental effects from the impurities in the ordinary tap water.
- Food Industry. Apparently this can reduce costs other than using heat-treatment processes.